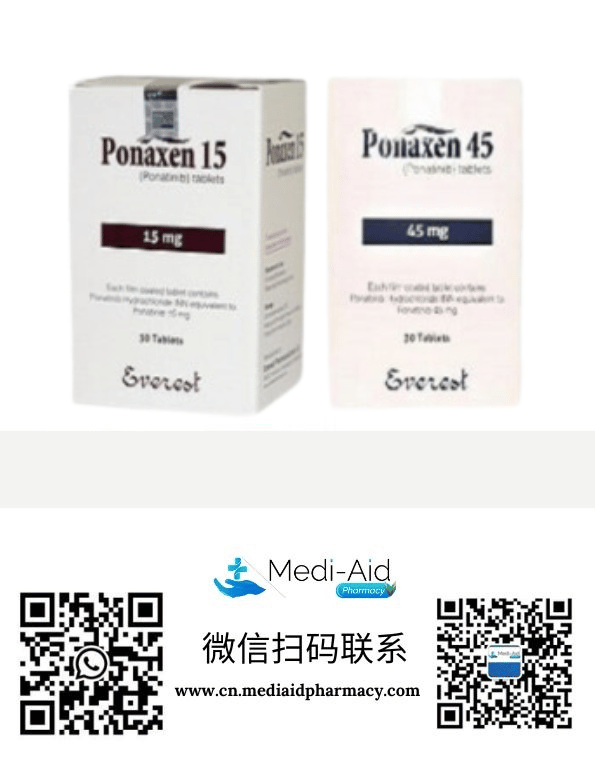
【product description】
Ponatinib is a third-generation BCR-ABL tyrosine kinase inhibitor (TKIs). On December 14, 2012, ponatinib was licensed by the US FDA to go on sale after fast-track approval for the treatment of chronic myelogenous leukemia (CML) with ABL T315I mutation or acute lymphoblastic leukemia (ALL) with Philadelphia chromosome positive (Ph+) It can also be used for the treatment of CML or ALL that is resistant or intolerant to previous tyrosine kinase inhibitors (TKI).
[Indications]
It is suitable for the treatment of adult patients with chronic myelogenous leukemia (CML) in chronic phase, accelerated phase, or blast phase who are resistant or intolerant to previous tyrosine kinase inhibitor therapy.
【Dosage】
The recommended dose is 45 mg once daily, orally with or without food;
treatment should be continued as long as there is no evidence of disease progression or unacceptable toxicity;
dose adjustment or interruption for hematological and non-hematological toxicity.





商品評價
目前沒有評價。