Lenvatinib (Lenvatinib, also translated as: Lenvatinib), research code: E7080, developed by Eisai, is a multi-target receptor tyrosine kinase (RTK) inhibitor, Inhibits VEGFR1, VEGFR2, and VEGFR3, as well as other RTKs associated with pathological neovascularization, tumor growth, and cancer progression, including fibroblast growth factor (FGF) receptors FGFR1, 2, 3, 4, and platelet-derived growth factor Receptor alpha (PDGFRα), KIT and RET.
In 2015, the US FDA and the European Medicines Agency EMA approved lenvatinib for the treatment of aggressive, locally advanced or metastatic differentiated thyroid cancer. In 2016, the US FDA and the European EMA successively approved lenvatinib combined with everolimus for the treatment of advanced renal cell carcinoma. In March 2018, lenvatinib was approved in Japan for the first-line treatment of patients with unresectable hepatocellular carcinoma (HCC). This is the first time the drug has been approved for indications for the treatment of hepatocellular carcinoma in the world, and it is also the first innovative systemic therapy for liver cancer that can be used for front-line treatment in Japan in 10 years.
In the treatment of advanced renal cell carcinoma, compared with everolimus alone, the combination of lenvatinib and everolimus can prolong the progression-free survival (PFS) of patients, and improve the objective response rate and overall survival rate of patients. The results of the study showed that the median PFS of patients in the combination group of lenvatinib and everolimus was 14.6 months, and the median PFS of patients in the everolimus monotherapy group was 5.5 months, an increase of nearly 3 times. Compared with the single drug group, the risk of disease progression or death in the combined drug group was reduced by 63%. The objective response rate was 37% in the combination group and 6% in the monotherapy group. The overall survival (OS) of patients in the combined drug group was 25.5 months, and that in the single drug group was 15.4 months.
For the first-line treatment of unresectable hepatocellular carcinoma, the phase III clinical trial REFLECT study of lenvatinib released by the American Society of Clinical Oncology ASCO at the 2017 meeting showed that in terms of the primary endpoint, the overall survival (OS) of the lenvatinib group ) has a tendency to prolong compared with the sorafenib group; in terms of secondary endpoints, the progression-free survival (PFS) of lenvatinib is twice that of sorafenib (7.4 vs 3.7 months), and the median time to disease progression ( mTTP) and objective response rate surpassed sorafenib (8.9 vs 3.7 months, 24% vs 9%). The REFLECT study is a global multi-center randomized, open-label, non-inferiority (NI) clinical study. The great success of lenvatinib in the REFLECT study may change the status quo of liver cancer drug treatment in the past decade.
【Indications】
1 Differentiated thyroid cancer (DTC): suitable for the treatment of patients with locally recurrent or metastatic iodine-refractory differentiated thyroid cancer
2 Renal cell carcinoma (RCC)
- In combination with pembrolizumab for the first-line treatment of adults with advanced renal cell carcinoma (RCC)
- In combination with everolimus (everolimus), for patients with advanced renal cell carcinoma who have received one anti-angiogenic therapy
3 Hepatocellular carcinoma (HCC): For the first-line treatment of patients with unresectable hepatocellular carcinoma (HCC)
4 Endometrial cancer (EC): used in combination with pembrolizumab for the treatment of patients with advanced endometrial cancer who are not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) Disease progression after systemic therapy and not amenable to radical surgery or radiotherapy
[Usage and Dosage]
Monotherapy:
1. Differentiated thyroid cancer (DTC): The recommended dose is 24 mg, once a day
2. Hepatocellular carcinoma (HCC):
The recommended dose is based on actual body weight:
Patients with a body weight greater than or equal to 60 kg : Oral, once a day, 12 mg each time
·Patients weighing less than 60 kg: Oral, once a day, 8 mg each time
Combination therapy:
1. Endometrial cancer (EC): The recommended dose is 20 mg orally, once a day , combined with pembrolizumab 200 mg intravenous infusion over 30 minutes, once every 3 weeks
2. Renal cell carcinoma (RCC): The recommended dose is
- 20 mg orally, once a day, combined with pembrolizumab 200 mg intravenous infusion over 30 minutes, once every 3 weeks
- 18mg orally once a day, combined with everolimus 5mg orally, once a day to adjust the recommended daily dose for some patients with renal or hepatic impairment
Lenvatinib must be taken at the same time each day. If you forget to take a dose and do not take it within 12 hours, skip that dose and take the next dose at the scheduled time.
Lenvatinib capsules should be swallowed whole, or dissolved in a small glass of liquid before taking. Capsules can be filled with a tablespoon of water or apple juice in a glass (capsules do not need to be opened or crushed). Let the capsules sit in the liquid for at least 10 minutes, then drink the liquid after stirring for at least 3 minutes. After drinking, add an equal amount (one tablespoon) of water or apple juice to the glass, mix well and drink the remaining liquid.
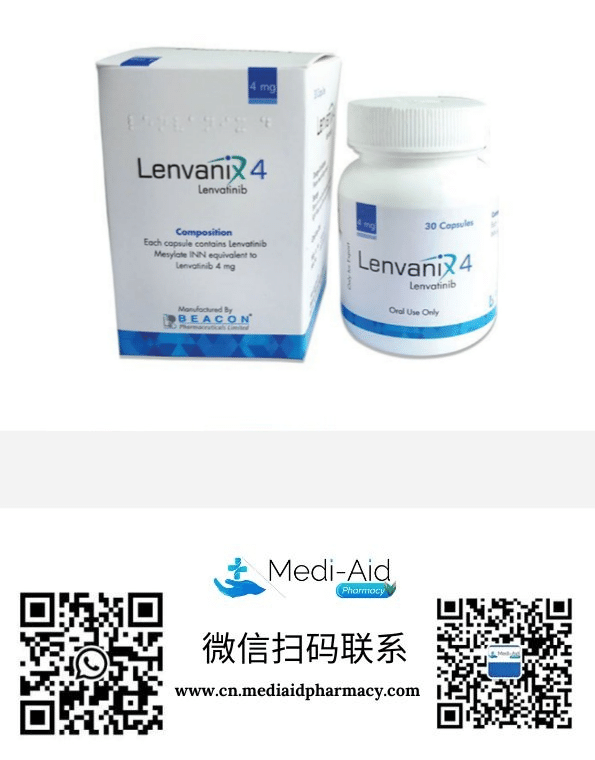

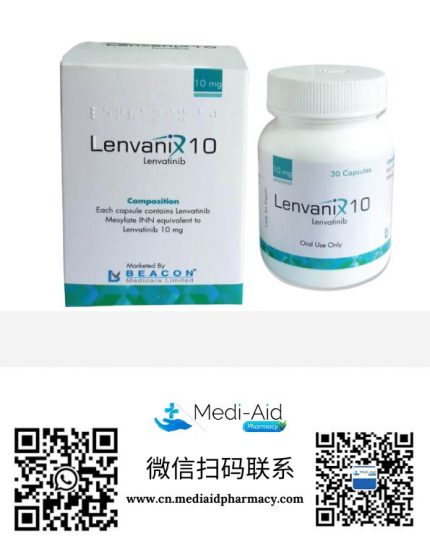
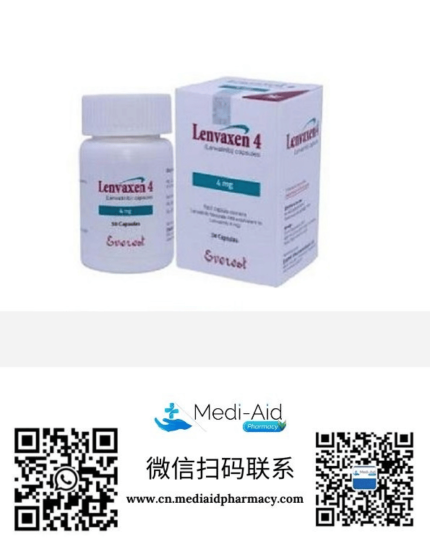
孟加拉珠峰Everest制药-1-430x549.png)



商品評價
目前沒有評價。